The progeny of the neural crest builds our teeth
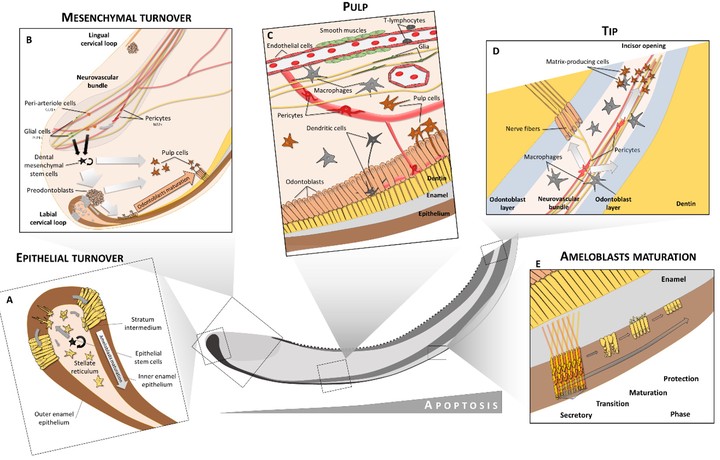
Mammalian teeth are built of many cell types including those producing hard matrix – odontoblasts making dentin, ameloblasts producing enamel and cementoblasts generating tooth cement. Numerous cell subtypes reside inside of the soft dental pulp and in the surrounding tooth follicle. Vessels and sensory nerves complete the structure of a tooth. Many of these dental cells are neural crest-derived and commit to dental-specific fates because of the crosstalk between the oral epithelium and the neural crest-derived mesenchyme, resulting in a forming tooth germ. For instance, during this process, the exact specification and differentiation of the odontoblast lineage has not been fully clarified, and the eventual fating of mesenchymal neural crest progenitors was not fully addressed. As a part of collaboration with laboratories of Kaj Fried and Jan Krivanek, we currently work on the heterogeneity of cell populations building our teeth, aiming to identify region- and tooth type-specific stem cells and define novel regenerative strategies. Recently, we utilized single cell transcriptomics to address the cell type heterogeneity in human and mouse teeth (please see Krivanek et al., Nature Communications 2020). In that study, we reported an unappreciated cellular complexity of the continuously-growing mouse incisor, which suggests a coherent model of cell dynamics enabling un-arrested growth. This model relies on spatially-restricted stem, progenitor and differentiated populations in the epithelial and mesenchymal compartments underlying the coordinated expansion of two major branches of pulpal cells and diverse epithelial subtypes. Further comparisons of human and mouse teeth yield both parallels and differences in tissue heterogeneity and highlight the specifics behind growing and non-growing modes. Despite being similar at a coarse level, mouse and human teeth reveal molecular differences and species-specific cell subtypes suggesting possible evolutionary divergence. Today, we continue this work and attempt to investigate phenotypic plasticity of cell types within teeth resulting from regenerative or infection-related stimuli.