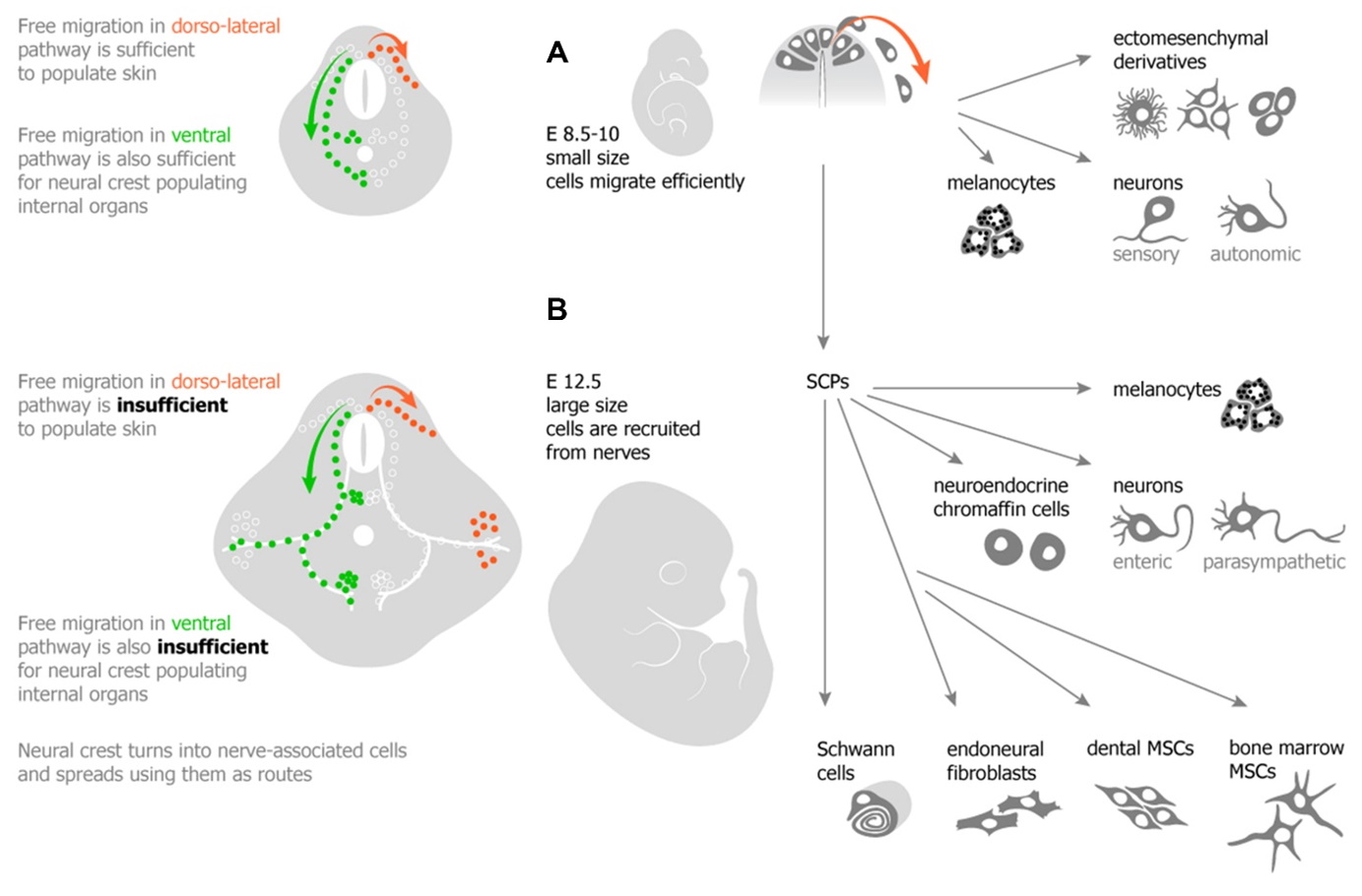
Multipotency of SCPs. Role of SCPs in development of sympathoadrenal system
Recently, we have discovered an entirely new phenomenon in developmental biology – targeted recruitment and subsequent differentiation of multipotent cells from the pervasive peripheral nerves. In brief, during recent years, one of the aims of our research was to define the source of the late embryonic and adult neural crest to harness the regenerative power of this cellular population. With the help of various methods, we revealed a new principle allowing embryos to position cells during histogenesis: nerve-associated glia and neural crest-like progenitor pools undergo nerve-regulated migration and differentiation into peripheral autonomic neurons (Dyachuk et al., Science 2014), neuroendocrine chromaffin cells (Furlan et al., Science 2017), melanocytes (Adameyko et al., Cell 2019) and different types of mesenchymal cells (Kaukua and Khatibi et al., Nature 2014). Our first breakthrough came in 2009 (Cell) and followed in 2012 (Development) demonstrating that Schwann cell precursors residing along growing peripheral nerves give rise to melanocytes (pigment cells in skin). Today, many biological questions in the lab are focused around the idea that peripheral nerves are essential for the development of organs; they contribute multipotent precursor cells to local tissues and serve as delivery routes for universal progenitors giving rise to numerous cell types. We apply single cell transcriptomics, color multiplexing in genetic tracing as well as functional studies in mouse, chick and zebrafish to address the relationships and molecular similarities between peripheral glial cells and migratory neural crest populations. Our single cell approach coupled to powerful validation systems allows investigating gene regulatory networks that drive differentiation of the neural crest and nerve-associate multipotent Schwann cell precursors.
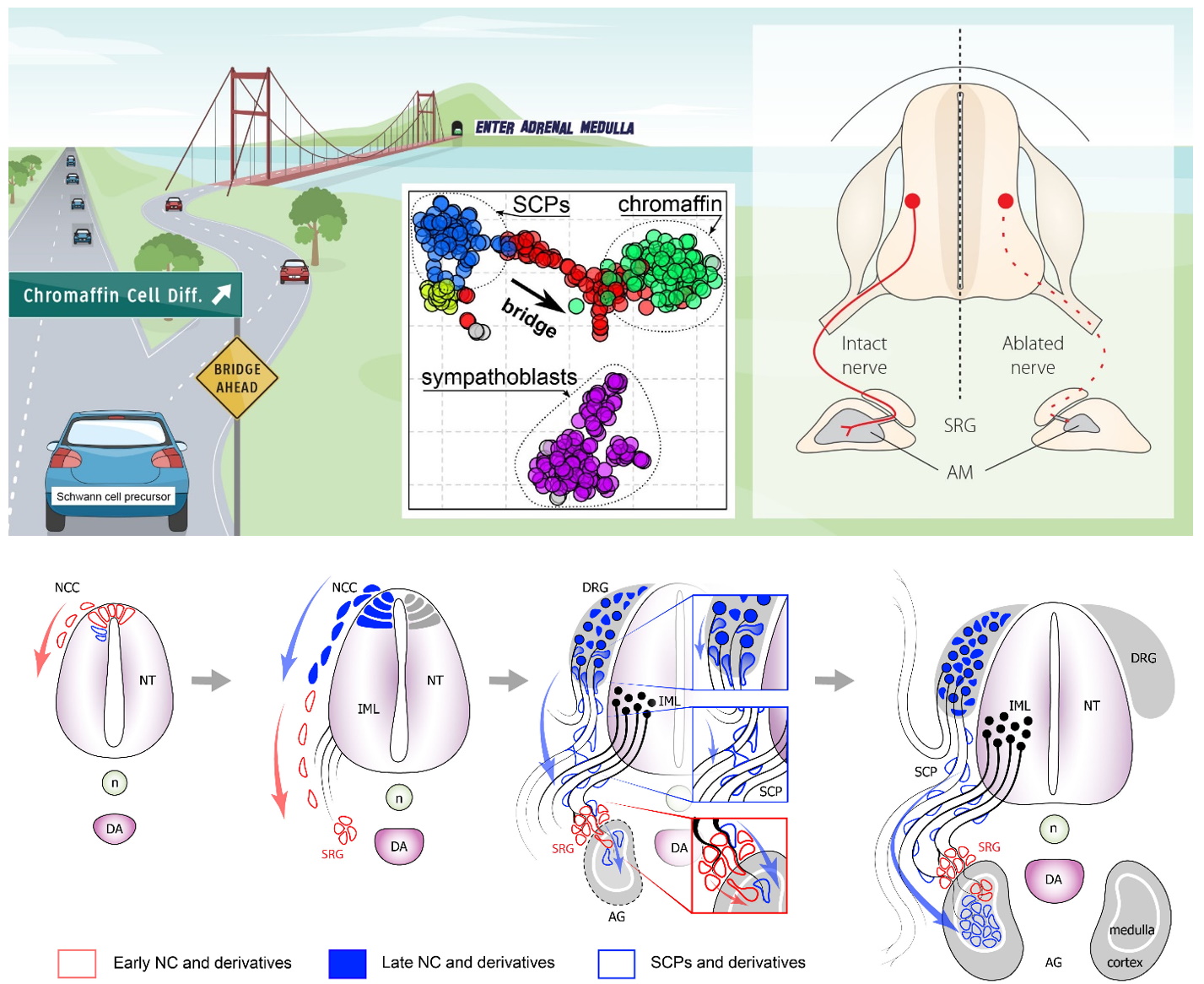
We recently explored sympathoadrenal development and discovered new Schwann cell precursor-dependent cellular origin of chromaffin cells using single cell transcriptomics (Furlan et al. Science 2017). Next, we started to investigate the malignancies arising from the sympathoadrenal origin such as neuroblastoma, pheochromocytoma and paraganglioma to relate intra-tumor heterogeneity to the potential cell of origin. In our preliminary data, we observed that these tumors are highly heterogeneous and resemble stages of the neural crest differentiation into chromaffin, glomus, or sympathetic cells. Clarifying the role of the nerve-associated Schwann cell precursors in the development of sympathoadrenal organs and corresponding malignancies might help to reveal the causes of disease heterogeneity. Creating “comparative identity maps” using single cell analyses of tumors and healthy progenitor populations will allow us to identify the precise developmental and adult origin cell types and corresponding differentiation statuses for different subtypes of neural crest tumors.
Video on YouTube about our work: https://www.youtube.com/watch?v=slZokCHgqV0 Key papers: https://www.ncbi.nlm.nih.gov/pubmed/28684471 “Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla” Science 2017.
https://www.ncbi.nlm.nih.gov/pubmed/24925909 “Neurodevelopment. Parasympathetic neurons originate from nerve-associated peripheral glial progenitors” Science 2014.
https://www.ncbi.nlm.nih.gov/pubmed/28242477 “Nerve-associated neural crest: peripheral glial cells generate multiple fates in the body” Curr Opin Genet Dev. 2017.